Abstract
PURPOSE: There is strong evidence that genetic factors influence retinopathy of prematurity (ROP), a neovascular eye disease. It has been previously suggested that polymorphisms in the genes involved in beta-adrenergic receptor (ADRβ) pathways could protect against ROP. Antagonists for the ADRβ are actively tested in clinical trials for ROP treatment but not without controversy and safety concerns. This study was designed to assess if genetic variations in components of the ADRβ signaling pathways associate with risk of developing ROP.
DESIGN: An observational case-control targeted genetic analysis.
METHODS: A study was carried out on premature participants with (n=30) or without (n=34) ROP and full-term controls (n=20) which were divided into a discovery cohort and a validation cohort. ROP was defined using International Classification of Retinopathy of Prematurity criteria (ICROP). Targeted sequencing of 20 genes in the ADRβ pathways was performed on the discovery cohort. Polymerase chain reaction (PCR)/restriction enzyme analysis for some of the discovered ROP-associated variants was performed for validation of the results using the validation cohort.
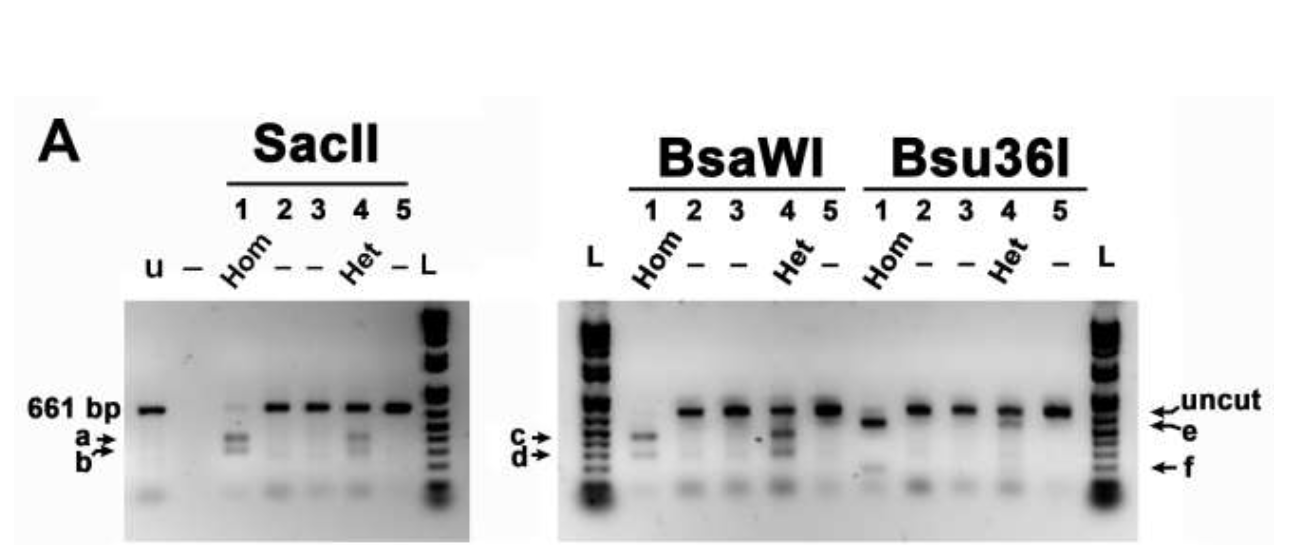
RESULTS: The discovery cohort revealed 543 bi-allelic variants within 20 genes of the ADRβ pathways. Ten single nucleotide variants (SNVs) in five genes including protein kinase A regulatory subunit 1α (PRKAR1A), rap guanine exchange factor 3 (RAPGEF3), adenylyl cyclase 4 (ADCY4), ADCY7, and ADCY9 were associated with ROP (p0.05). The most significant SNV was found in PRKAR1A (p=0.001). Multiple variants located in the 3'-untranslated region (3'UTR) of RAPGEF3 were also associated with ROP (p0.05). PCR/restriction enzyme analysis of the 3'UTR of RAPGEF3 methodologically validated these findings.
CONCLUSION: SNVs in PRKAR1A may represent protective factors while SNVs in RAPGEF3 may represent risk factors for ROP. PRKAR1α has previously been implicated in retinal vascular development while the RAPGEF3 product has a role in the maintenance of vascular barrier function, two processes important in ROP. Multicenter validation of these newly discovered risk factors could lead to valuable tools for predicting and preventing the development of severe ROP.
Journal
Year of Publication